
Halogens on the Right In the second column from the right side of the periodic table, you will find Group (Group XVII).This column is the home of the halogen …
Atomic and physical properties . . . Discusses trends in atomic radius, electronegativity, electron affinity and melting and boiling points of the Group 7 elements.


The typical physical properties of the halogens are described and discussed with reference to the data table. Another table describes the …



This page explores the trends in some atomic and physical properties of the Group 7 elements (the halogens) – fluorine, chlorine, bromine and iodine.
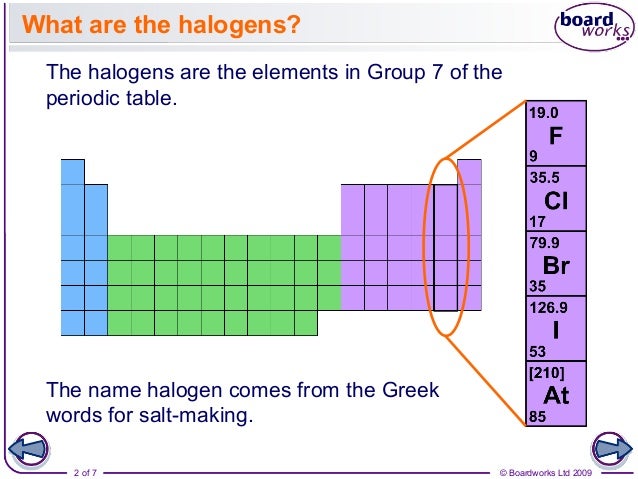
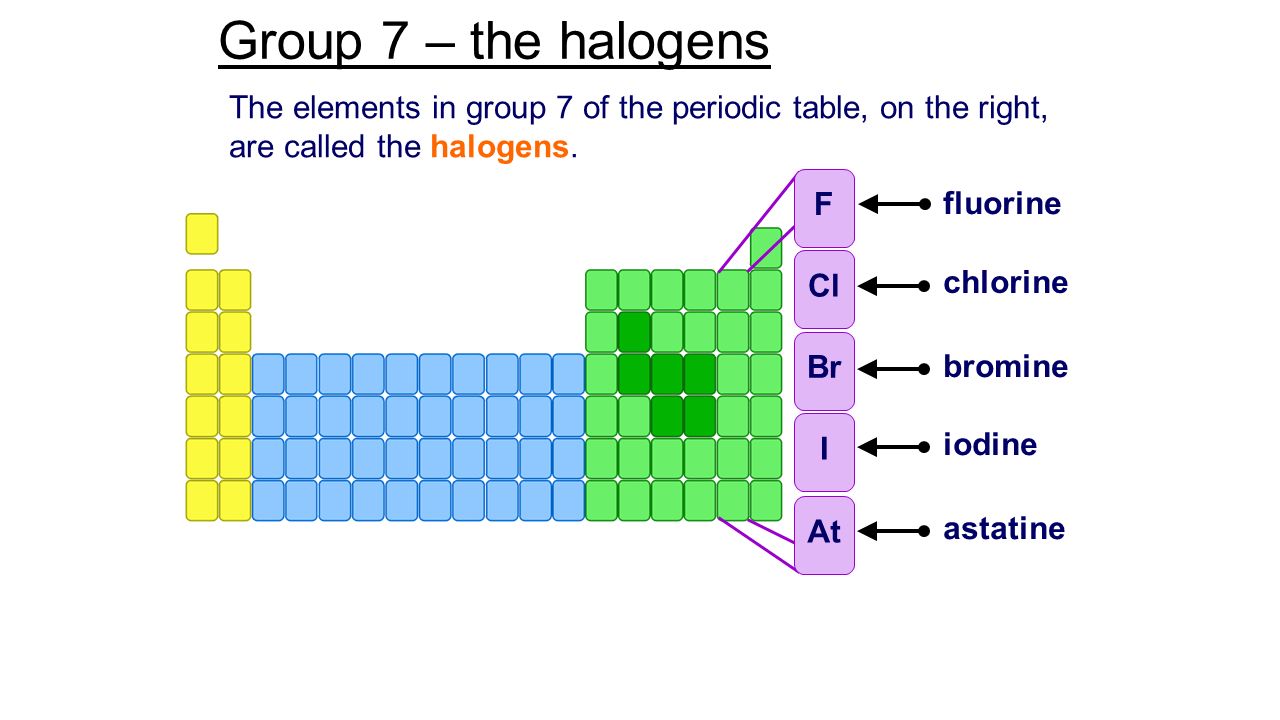
The halogens (/ ˈ h æ l ə dʒ ə n, ˈ h eɪ-, – l oʊ-, – ˌ dʒ ɛ n /) are a group in the periodic table consisting of five chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At).
The halogens are five non-metallic elements found in group 17 of the periodic table. The term “halogen” means “salt-former” and compounds containing halogens …
The halogens are the family of chemical elements that includes fluorine (atomic symbol F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At).
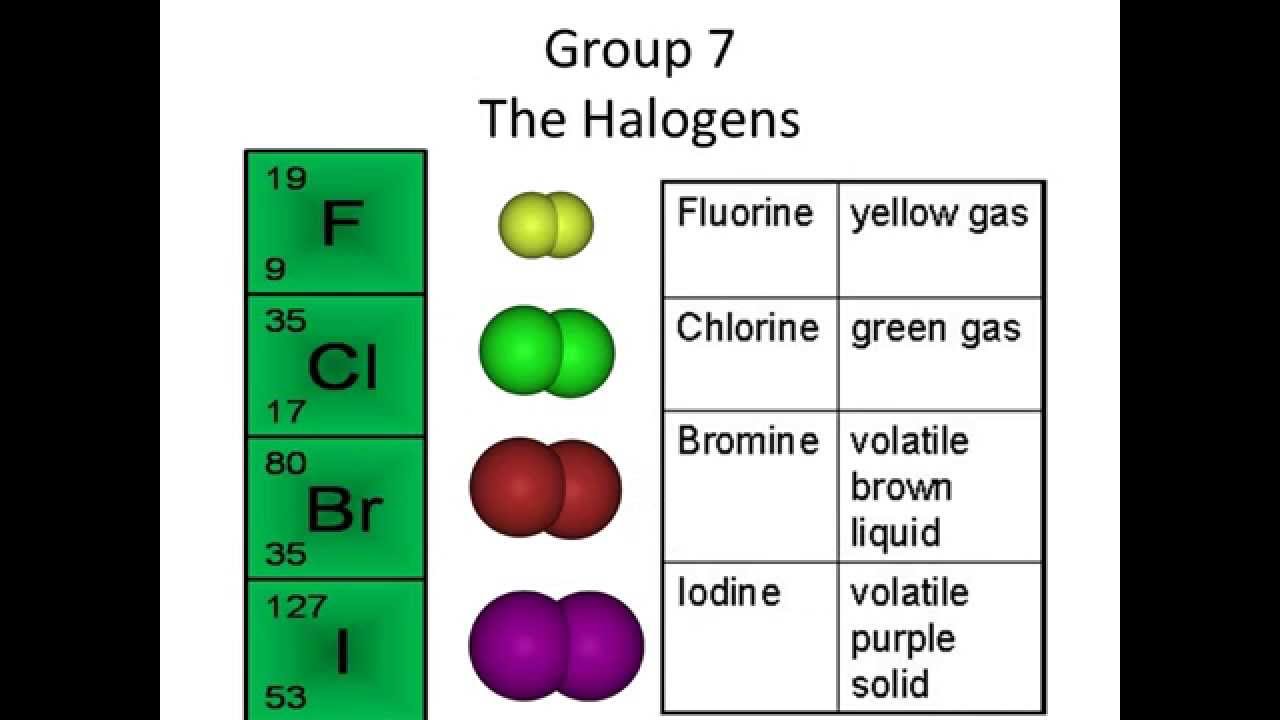

The physical properties of the Group 7 halogens – fluorine, chlorine, bromine, iodine and astatine the chemical displacement reactions of chlorine, bromine and iodine, explaining the reactivity trend of the Group VII halogen elements, the uses of the halogens, halide salts and halogen organochlorine compounds IGCSE/GCSE revision …
The halogens are the five chemical elements that make up Group 17 on the periodic table: fluorine, chlorine, bromine, iodine, and astatine.
Free teaching notes for group 7 chemistry at O level, IGCSE and GCSE